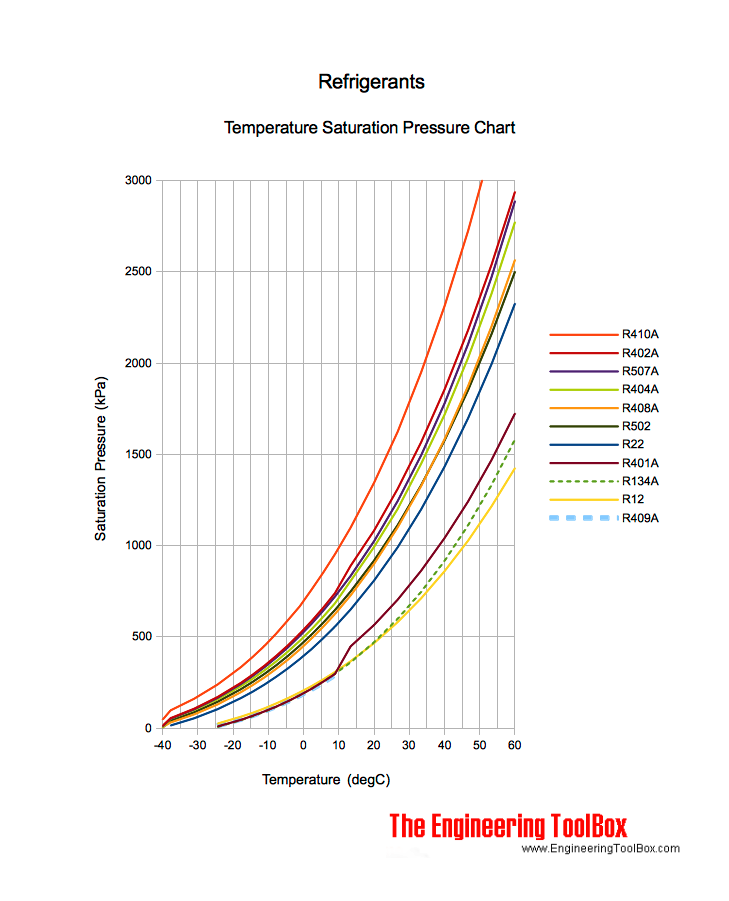
If the same experiment is conducted by him her in austin where mean room temperature and pressure is 21 and 1 014 bar the result of the experiments might significantly vary.
Value of room temperature and pressure.
When it occupies a larger volume it exerts a lower pressure assuming the amount of gas and the temperature do not change.
Ntp is defined as air at 20 degrees c 293 15 k 68 degrees f and 1 atm 101 325 kn m 2 101 325 kpa of pressure.
Volume pressure data for an air sample at room temperature are graphed in figure 5.
Satp standard ambient temperature and pressure is also used in chemistry as a reference.
The relationship between pressure and temperature of a gas is stated by gay lussac s pressure temperature law.
When a gas occupies a smaller volume it exerts a higher pressure.
Human comfort can extend beyond this range depending on humidity air circulation and other factors.
Mean room temperature and pressure in london is 12 and 1 015 bar.
1 mole of any gas occupies 22 4 dm 3 at stp standard temperature and pressure taken as 0 c and 1 atmosphere pressure.
For ordinary temperatures another common value is ntp which stands for normal temperature and pressure.
59 00 f and 101 325 kpa.
This standard is also called normal temperature and pressure abbreviated as ntp.
The value of r the ideal gas constant depends on the units chosen for pressure temperature and volume in the ideal gas equation.
Nist uses a temperature of 20 c 293 15 k 68 f and an absolute pressure of 1 atm 14 696 psi 101 325 kpa.
Satp standard ambient temperature and pressure.
Colloquially room temperature is the range of air temperatures that most people prefer for indoor settings which feel comfortable when wearing typical indoor clothing.
While stp is standard temperature and pressure not many measured processes occur when it s freezing.
These figures are actually only true for an ideal gas and we ll have a look at where they come from.
Satp standard ambient temperature and pressure is a reference with temperature of 25 o c 298 15 k and pressure of 101 325 kpa.
At these conditions the volume of 1 mol of a gas is 24 4651 liters.
The rates of evaporation and condensation over time for a system such as this are shown graphically in figure pageindex 3.
At this point the pressure over the liquid stops increasing and remains constant at a particular value that is characteristic of the liquid at a given temperature.
In certain fields like science and engineering and within a particular context room temperature can mean different agreed.
It is necessary to use kelvin for the temperature and it is conventional to use the si unit of liters for the volume.